Brussels – Tomorrow, the European Commission will unveil in the Strasbourg hemicycle the draft law on critical medicines, which was clamoured for by member states more than a year ago. Now, as the continent’s arms race takes its first steps, the growing shortages of essential medicines are in danger of taking a back seat. And the EU’s dangerous dependence on medicines risks being “the Achilles heel of its defense strategy,” suggest 11 member countries in an editorial published by Euronews.
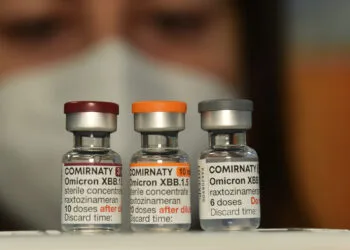
Currently the European Union imports from India and China between 60 and 80 per cent of the active pharmaceutical ingredients (APIs) used for generic drugs in the 27 member countries. Nearly 90 per cent of the world’s antibiotics are produced in Asia. In an increasingly aggressive geopolitical environment, “it is easy for foreign actors to turn this dependence into a critical vulnerability,” highlight the health ministers of Belgium, Spain, Germany, the Czech Republic, Greece, Latvia, Estonia, Lithuania, Portugal, Slovenia, and Cyprus. It is no longer dystopian to imagine a scenario in which the antibiotic supply chain is suddenly disrupted.
Shortages of essential medicines in some member countries already exist and are nothing new, which is why Brussels drew up the first Union’s list of critical medicines last year and established an alliance for essential medicines. Ursula von der Leyen has included the Critical Medicines Act in the policy guidelines of her second term, and the EU Commissioner for Health, Hungary’s Olivér Várhelyi, has promised to present the proposal in the first hundred days after taking office.

The co-legislators are waiting at the gates. Tomorrow, MEPs will press the Commission on measures designed to bring manufacturing back to Europe and facilitate joint procurement. The 11 capitals that addressed the information network have speculated that “part of the funding” from the Critical Medicines Act should be“integrated into the EU’s broader defence spending plans, including the financial mechanisms of the new defence package,” Rearm Europe. After all, the states insist, “without essential medicines, Europe’s defense capabilities are compromised.”
On a parallel track, Belgium, Cyprus, France, Greece, Italy, Malta, Portugal, Romania, Spain, and Hungary have circulated among the 27 a non-paper (an informal document), a kind of “manifesto” for “ambitious” legislation to address shortages of essential medicines in the common market. This phenomenon has multiple and complex causes, but it is largely due to the progressive collapse of domestic production in favour of offshoring, which exposes Europe to potential crises.
In the exploratory document, the capitals advocate the development of an industry-wide list of vulnerable medicines as early as 2025 to “prioritize support and strengthen EU-based manufacturing and supplier diversification efforts.” A list of pharmaceutical shortages should be drawn up by the European Medicines Agency (EMA) and experts from member countries. A kind of first step for a specific investment plan “with high cooperation and coordination at the EU level,” to be developed “perfecting the EU state aid rules and financial framework.” Complementarily, there is an urgent need to “recognize the importance of supply chain diversification”: according to member countries, Brussels should “assess the prospects of potential international partnerships” on essential medicines.
The importance of joint procurement is certainly among the lessons left behind by the COVID-19 pandemic. Again, member countries hope the new Critical Medicines Act will define an “ambitious framework” for joint public procurement. “Increasing manufacturing capacity in Europe is a necessity, but it will have limited impact if we are not able to purchase differently,” the non-paper reads.
All of these recommendations, which were also put down on paper during 2024 by the Essential Medicines Alliance sought by von der Leyen, will have to be included in some way in the proposal that Varhelyi will present to MEPs in plenary tomorrow. The European Commission’s proposal will then end up on the table of the relevant ministers from the member countries and the EU Parliament’s new Committee on Public Health (SANT), who will help shape the law’s final form.
English version by the Translation Service of Withub




![Il presidente della Repubblica, Sergio Mattarella, con la presidente della Commissione europea, Ursula von der Leyen [Bruxelles, 21 maggio 2025. Foto: Emanuele Bonini per Eunews]](https://www.eunews.it/wp-content/uploads/2025/05/mattarella-vdl-120x86.png)


![Il presidente della Repubblica, Sergio Mattarella (sinistra), con il presidente del Consiglio Europeo, Antonio Costa [Bruxelles, 20 maggio 2025. Foto: Quirinale]](https://www.eunews.it/wp-content/uploads/2025/05/mattarella-costa-120x86.jpeg)